Abstract
Introduction: Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the consolidation therapy with the highest chance of sustained remission for most patients (pts) with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). However, relapse occurs in up to 40% of pts and adversely affects prognosis. Currently, post-HSCT relapse strategies include intensive chemotherapy, hypomethylating agents (HMA), as well as targeted therapy, e.g. FLT3-inhibitors frequently combined with donor lymphocyte infusions (DLIs). Here we investigated combinations of HMA and venetoclax (VEN) as salvage options for relapse after alloHSCT.
Methods: 2 MDS and 24 AML pts (median age 60 [range 38-73] years) relapsed after a median of 6.2 (range 1.6-55.5) months (mo) after 1st (n=22) or 2nd (n=4) HSCT. Conditioning regimens were myeloablative (n=5) or non-myeloablative (n=21). Remission status at HSCT was composite complete remission (CRc; including CR, CR with incomplete recovery [CRi] and morphologic leukemia free state [MLFS]; 50%), partial remission or stable disease (12%), and refractory (38%). While 22 pts had hematological relapse, 4 pts showed either combined (n=3) or sole (n=1) extramedullary disease. All pts subsequently received HMA/VEN-based therapies (azacitidine/venetoclax [n=22], azacitidne/venetoclax/gilteritinib [n=3] or decitabine/venetoclax [n=1]) with concomitant DLI in 13 pts. Prior to HMA/VEN, 13 pts received a different relapse therapy including azacitidine [n=7], gilteritinib [n=2], radiation [n=1], an investigational therapy with an IRAK4 inhibitor [n=1], or a combination therapy with azacitidine and pevonedistat [n=1] or tagraxofusp [n=1]. European LeukemiaNET 2022 risk at the timepoint of relapse was favorable (n=2), intermediate (n=4) or adverse (n=20). Median follow-up since HMA/VEN start was 6.4 mo.
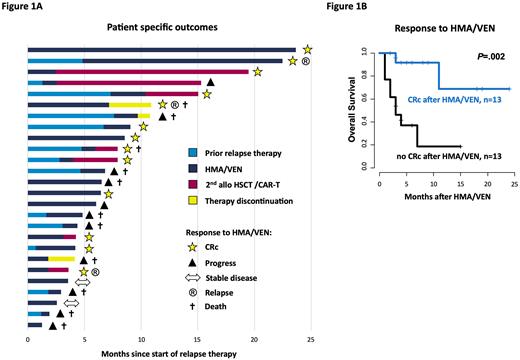
Results: HMA/VEN was well tolerated and administered in an outpatient setting in 20/26 pts (77%). The most common adverse effects were expected and included neutropenia CTCAE 3/4° in 25/26 pts (96%) and thrombocytopenia CTCAE 3/4° in 22/26 pts (85%). Eight pts (31%) developed at least one episode of febrile neutropenia. Four pts (15%) showed signs of afebrile infections and were hospitalized. Thirteen pts (50%) achieved a CRc (CR [n=3], CRi [n=8], MLFS [n=2]) under HMA/VEN therapy (Figure 1A), with a median time to 1st and best morphological response of 1.0 and 2.1 mo, respectively. Later relapse (>12 mo after HSCT) and a lower number of prior therapies before HMA/VEN (≤2 vs. >2 lines) associated with improved outcomes (cumulative incidence of achieving a CRc: P=.004 and P=.04, and OS: P=.02 and P=.02, respectively). Six of 13 pts (46%), who received a prior non-HMA/VEN therapy for the current relapse achieved a CRc after switching to HMA/VEN. Pts achieving a CRc had a significantly longer OS compared to pts with no CRc (P=.002; Figure 1B). Of the pts with CRc, 7 continued HMA/VEN with a median response duration of 7.2 mo (6/7 pts are still on treatment at last follow-up), 5 received a 2nd HSCT and 1 an experimental CART-therapy. For those that could be bridged to a 2nd HSCT outcomes were beneficial with 4/5 pts (80%) alive and disease-free (median follow-up since 2nd HSCT: 4.3 months). One patient with CART-therapy suffered from a 2nd relapse 1.7 months after treatment and is currently again salvaged with HMA/VEN. Two of 13 pts (15%) died after initial CRc, 1 due to non-relapse mortality of a 2nd HSCT and 1 by losing treatment response. Of the 13 pts who did not achieve a CRc, 1 patient could be bridged to 2nd HSCT with a post-HSCT relapse free survival of 12 mo. In the remaining 12 non-CRc pts median OS since start of HMA/VEN was 2.9 mo (range 0.7-6.5 mo) and 9/12 pts (75%) died due to progressive disease. Regarding the molecular risk profile, none of the TP53 mutated pts (n=3) responded, whereas pts with IDH1/2, NPM1, or myelodysplasia-related gene mutations, i.e.ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2, had mixed responses (2/5 pts [40%], 1/3 pts [33%], and 8/15 pts [53%], respectively).
Conclusion: HMA/VEN is a promising and well tolerated treatment strategy for MDS and AML pts relapsing after HSCT with a CRc of 50% and potential long-term survival. Especially pts that could be bridged to a 2nd HSCT benefited from the treatment. However, in pts with a disease resistant to HMA/VEN, outcome was very poor with a median OS of only 2.9 mo.
Disclosures
Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Herling:EDO-Mundipharma: Honoraria, Research Funding; Janpix: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Merz:BMS Celgene: Honoraria; Janssen: Honoraria. Metzeler:Daiichi Sankyo: Honoraria; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy, Honoraria, Research Funding; Curis: Research Funding; Astellas: Honoraria; AbbVie: Honoraria. Jentzsch:Pfizer: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria. Schwind:Novartis: Honoraria.
OffLabel Disclosure:
Venetoclax for relapsed AML after allogeneic HSCT
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal